Gives genomics a shared evidence language.
Every variant. Every pipeline. Every institution.
QuantBayes standardises how evidence strength is expressed, without changing how variants are discovered.
QuantBayes adopts a shared evidence standard. Foundational tools must be unrestricted so results can move safely between laboratories, hospitals, and national programmes.
Read moreQuantBayes does not estimate pathogenicity.
It does not replace analysis pipelines. It connects them.
QuantBayes measures how complete and verifiable the available evidence is for any variant selected by an upstream workflow. It applies a universal statistical standard that behaves identically across laboratories, platforms, and countries. Existing technologies remain unchanged while gaining a transparent, reproducible evidence scale that can move across institutions.
A strongly supported variant might score 0.95 with a 95 percent credible interval from 0.91 to 0.98. A weaker result might score 0.32 with a wider interval.
These values are independent of the pipeline that produced the candidate variant. This separation of variant discovery from evidence assessment provides a common, auditable evidence language for genomics.
QuantBayes is designed as non-diagnostic infrastructure suitable for regulated environments.
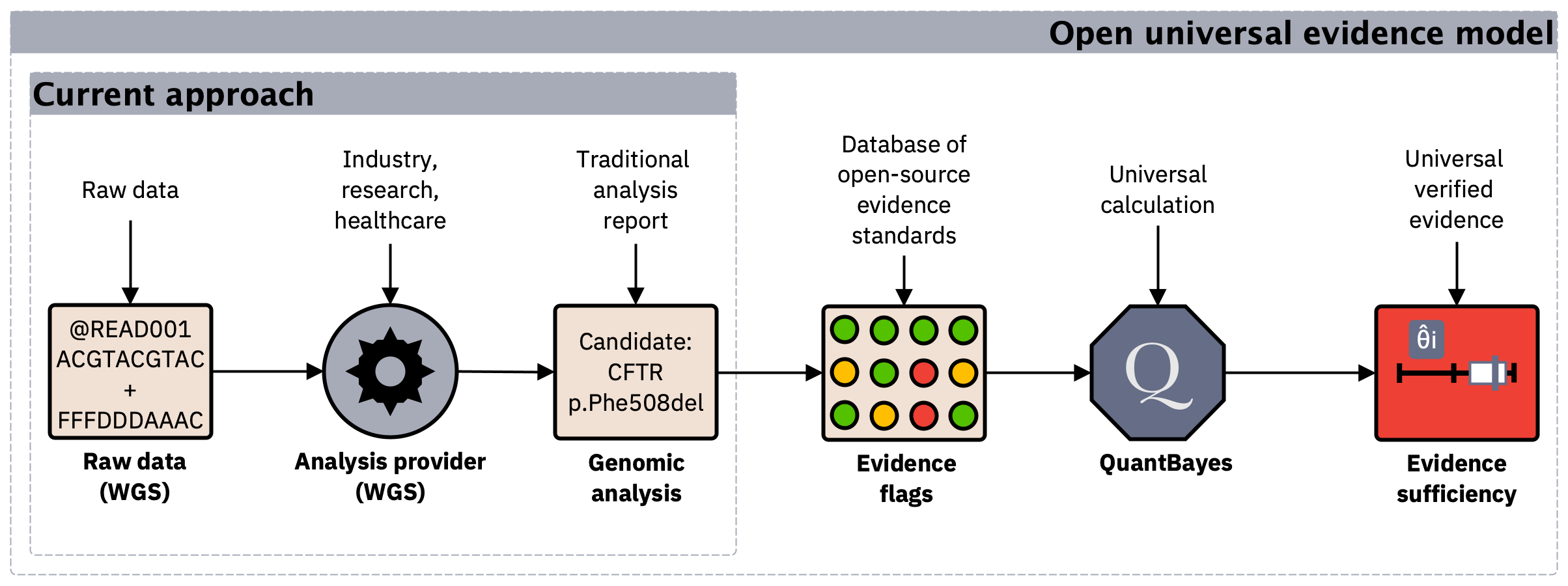 Figure 1. From siloed workflows to a universal evidence model.
Figure 1. From siloed workflows to a universal evidence model.
Downloads
| OS | File | SHA256 |
|---|---|---|
| R package | quantbayes_v0.1.0_R.tar.gz | dfa919d1848070cf8125d49eb029dbfd49d09558cd84deb7d50c1a6c188f1f63 |
| Linux x86_64 | quantbayes_v1.0.0_linux_x86_64.tar.gz | 440c5d92354285a5b7790d19f017205a849ecb76a4fc80804f726d420f06080d |
| macOS universal | quantbayes_v1.0.0_macos_universal.tar.gz | ab8824f0c29fce57328a3037677dbc20768b5a9d388fda1215362d1d3b99291e |
| macOS x86_64 | quantbayes_v1.0.0_macos_x86_64.tar.gz | c15d4ac299d4f557941b1a8518e75c44fd026cf9908edf8e85475b9f9e056035 |
R package installation
Source: cran.r-project quantbayes (for installation see below).
Installation
QuantBayes is distributed as a standalone binary and as an R package. The binary runs directly from the extracted directory and requires no system installation or administrator privileges. It is compatible with macOS, Linux, HPC environments, and shared compute clusters.
MacOS and Linux binary installation
Download verify checksums and extract:
shasum -a 256 quantbayes_v1.0.0_*.tar.gz
tar -xzf quantbayes_v1.0.0_<platform>.tar.gz
cd quantbayes
Run directly:
./quantbayes example_data/test_matrix_01.txt --report
macOS note
Downloaded binaries may be quarantined by macOS. Remove the quarantine flag if prompted:
xattr -d com.apple.quarantine quantbayes
Linux and HPC note
QuantBayes can be used from any directory with execute permissions. Administrators may optionally expose it via an environment module:
module load quantbayes
R package installation
Install with R or RStudio directly from CRAN. For more information see the R vignette and R reference manual pages.
install.packages("quantbayes")
library(quantbayes)
Quick start
Human readable report:
./quantbayes example_data/test_matrix_01.txt --report --out sample1
Minimal run:
./quantbayes example_data/test_matrix_01.txt
JSON output:
./quantbayes example_data/test_matrix_01.txt --json
QuantBayes produces per variant summaries, global summaries, and optional reports.
Example: clinical genetics
Whole Genome Sequencing produced a ranked list of candidate variants using a standard prioritisation workflow. QuantBayes evaluated the independent evidence supporting each candidate using a binary evidence matrix derived from a published evidence standard.
For the leading candidate, the posterior evidence sufficiency \(\theta\) and its 95 percent credible interval were 0.731 (0.549 to 0.879), corresponding to the 99.88th percentile genome wide. These values describe evidence completeness, not pathogenicity.
| variant_id | k | m | theta mean | theta lower | theta upper | percentile |
|---|---|---|---|---|---|---|
| 7-117559592-CTT–_AR | 18 | 24 | 0.7308 | 0.5487 | 0.8793 | 99.875 |
| 6-72183475-CG-N_AR | 18 | 24 | 0.7308 | 0.5487 | 0.8793 | 99.875 |
| 1-14682421-G-A_AD | 17 | 24 | 0.6923 | 0.5061 | 0.8505 | 99.375 |
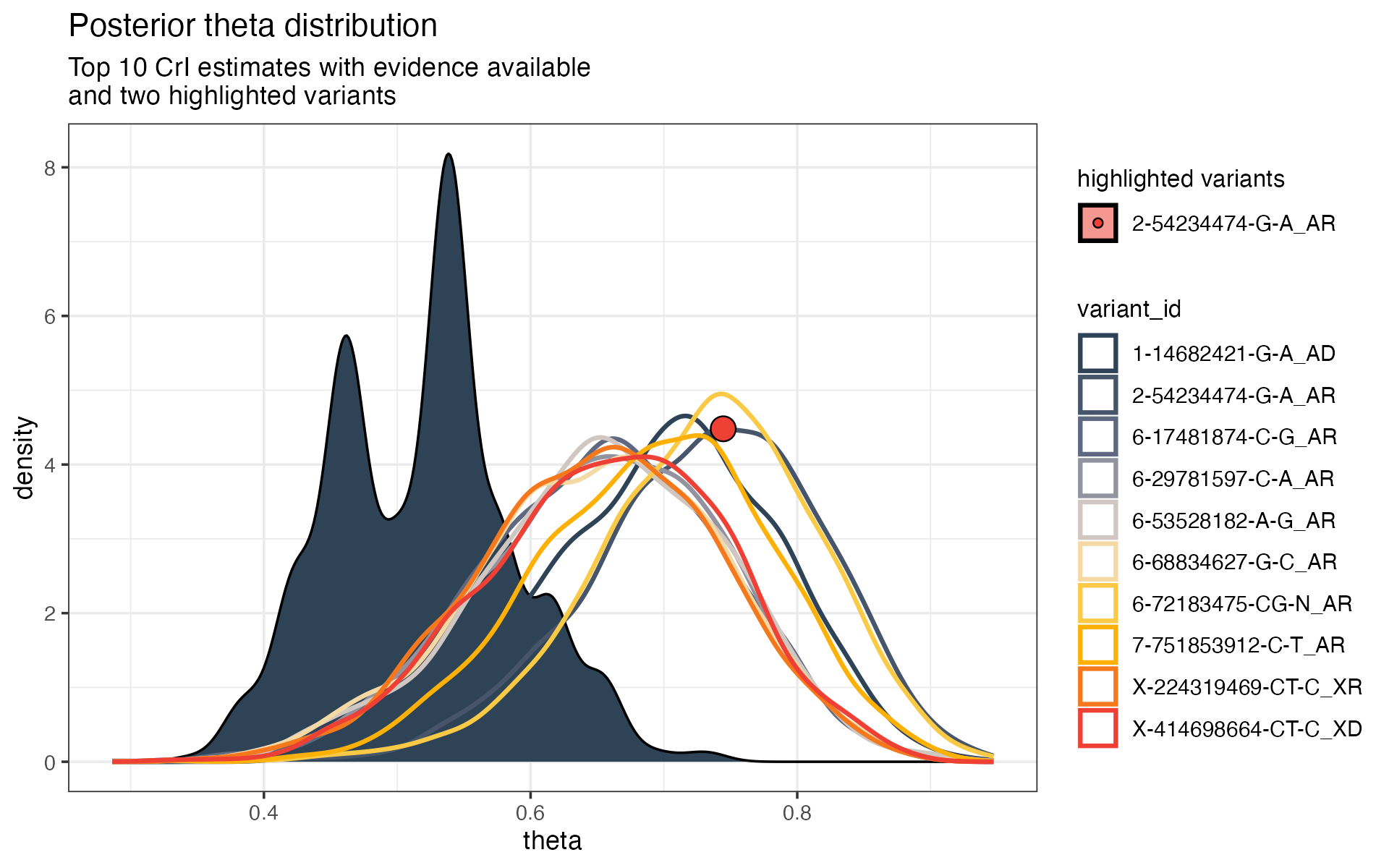 Figure 2. Global evidence sufficiency distribution with candidate overlay.
Figure 2. Global evidence sufficiency distribution with candidate overlay.
Method overview
QuantBayes models the number of supported evidence items (k) out of (m) using a Beta Binomial update:
\[\theta \mid k \sim \mathrm{Beta}(1 + k,\; 1 + m - k)\]The posterior mean, credible interval, and percentile provide a reproducible measure of evidence sufficiency. These values quantify completeness and verifiability of evidence and do not estimate disease causality.
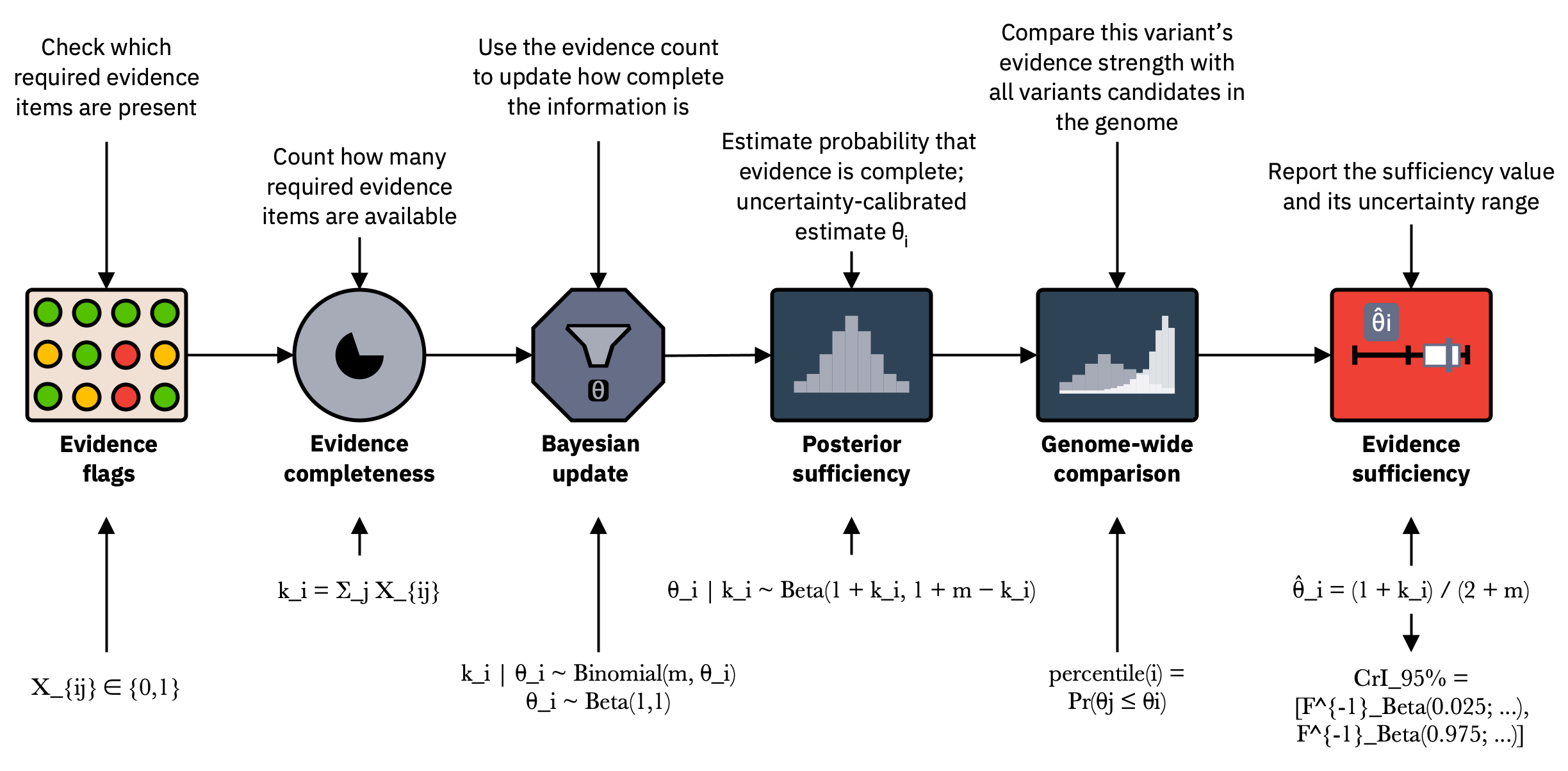 Figure 3. QuantBayes applied to a standardised evidence model.
Figure 3. QuantBayes applied to a standardised evidence model.
Citation
If you use QuantBayes please cite the software and the associated manuscript.
Manuscript
Quant Group, et al. A Bayesian model for quantifying genomic variant evidence sufficiency in Mendelian disease. medRxiv (2025). DOI: https://doi.org/10.64898/2025.12.02.25341503.
@article{QuantBayes2025quantgroup,
author = {Quant Group and Saadat, Ali and Lawless, Dylan},
title = {A Bayesian model for quantifying genomic variant evidence sufficiency in Mendelian disease},
elocation-id = {2025.12.02.25341503},
year = {2025},
doi = {10.64898/2025.12.02.25341503},
publisher = {Cold Spring Harbor Laboratory Press},
url = {https://www.medrxiv.org/content/early/2025/12/13/2025.12.02.25341503},
eprint = {https://www.medrxiv.org/content/early/2025/12/13/2025.12.02.25341503.full.pdf},
journal = {medRxiv}
}
R package (CRAN)
Lawless D. quantbayes: Bayesian Quantification of Evidence Sufficiency. Comprehensive R Archive Network (2025). https://CRAN.R-project.org/package=quantbayes
@article{quantbayes_cran,
title = {quantbayes: Bayesian Quantification of Evidence Sufficiency},
author = {Lawless, Dylan},
year = {2025},
note = {R package version 0.1.0},
url = {https://CRAN.R-project.org/package=quantbayes},
doi = {10.32614/CRAN.package.quantbayes},
journal = {Comprehensive R Archive Network},
organization = {Comprehensive R Archive Network}
}
Software release (Zenodo)
Lawless D. Bayesian Quantification of Evidence Sufficiency quantbayes Implementation. Zenodo (2025). DOI: https://doi.org/10.5281/zenodo.17919369.
@article{lawless2025_zenodo_quantbayes,
author = {Lawless, Dylan},
title = {Bayesian Quantification of Evidence Sufficiency quantbayes Implementation},
month = dec,
year = {2025},
journal = {Zenodo},
publisher = {Zenodo},
doi = {10.5281/zenodo.17919369},
url = {https://doi.org/10.5281/zenodo.17919369}
}
Data repository
We provide the universal macOS binary runs natively on both Intel x86_64 and Apple silicon arm64 systems and the R package source code. Release artefacts and documentation are hosted on Zenodo.
Licence
QuantBayes is released under the MIT Licence. This permits inspection, reproduction, and integration into commercial, research, and clinical workflows without restriction.


